

This means that there are UNEQUAL numbers at least one atom on each side of the arrow. It is an unbalanced equation (sometimes also called a skeleton equation). Therefore, we must finish our chemical reaction with as many atoms of each element as when we started.Įxample #1: Balance the following equation: H 2 + O 2 -> H 2O "Matter is neither created nor destroyed." "We may lay it down as an incontestible axiom, that, in all the operations of art and nature, nothing is created an equal quantity of matter exists both before and after the experiment the quality and quantity of the elements remain precisely the same and nothing takes place beyond changes and modifications in the combination of these elements." The law was discovered by Antoine Laurent Lavoisier (1743-94) and this is his formulation of it, translated into English in 1790 from the Traité élémentaire de Chimie (which was published in 1789): The Law of Conservation of Mass is the rationale for balancing a chemical equation. IMPORTANT DEFINITION: A balanced equation has equal numbers of each type of atom on each side of the equation. Making sure they are balanced must be done before the equation can be used in any chemically meaningful way.Īll chemical calculations you will see in other units must be done with a balanced equation.
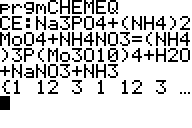
Discussion and Twenty Examples Probs 1-10 Probs 11-25 Probs 26-45 Probs 46-65 "Balancing by groups" problems Only the problems Return to Equations Menu Balance redox equations by sightĬhemical equations usually do not come already balanced.


 0 kommentar(er)
0 kommentar(er)
